
The Geology of the Peak District
The Peak District limestone was formed during the Carboniferous geological period. At this time, some 340 million years ago, Britain was part of a large continental landmass close to the equator.
In these tropical conditions rivers flowed into shallow warm seas teeming with primitive fish, molluscs, and coral reefs. Their Calcium shells combined with silt to form layer upon layer of Calcium Carbonate rich sediments several hundred metres thick. The fossil remains of these ancient plants and animals are easily recognisable in limestone.
These limestone layers were lifted, fractured, and folded by massive earth movements as the continental plates drifted apart. Ancient rivers deposited sands and silt layers over the limestone forming the Millstone Grit sandstones of the Northern Peak District.
Further earth movements and volcanic activity thrust the limestone upwards to form a dome whilst hot minerals flowed into the fractured rocks forming Galena (Lead Ore), Flourspar, Barytes, and Calcite veins. Erosion by rain, wind, and ice stripped away the Sandstone cap leaving limestone exposed to weathering and incised by numerous deep river valleys giving the Derbyshire Dales its familiar landscape. Water also began to carve out another landscape hidden deep underground.


Carboniferous limestone is a non-porous rock and therefore water is unable to soak through. However, the fractures, cracks, and joints formed in the rock due to earth movements allow water to penetrate limestone and can in turn be enlarged to form cave systems.
Rainwater dissolves some Carbon Dioxide gas from the atmosphere to become slightly acidic.
H2O + CO2 = H2CO3
Rain + Carbon Dioxide = weak Carbonic Acid
Rain becomes more acidic as it passes through soil and organic material. On reaching limestone the acid water can begin to dissolve the cracks and fissures. Streams also carry silt and sand from the surrounding Gritstone edges, which will erode and enlarge cracks further, thus a cave passage begins to form.
Caves tend to form, as they have at Poole's Cavern, down the dip of a slope, with water entering via Swallet Caves, or Shake Holes, on the higher slopes and draining to springs in the valley below. In Buxton these springs form the source of the Derbyshire River Wye.
Caves generally begin to form on water filled passages below the water table where limestone is saturated with water. As the passage enlarges and water levels drop, water only partially fills the cave, causing a keyhole shaped passage to form. This may further transform by the collapse of the passage walls or roof to form a very large cavern.
It is thought that this process of cave formation has been taking place at Poole's Cavern for at least two million years.
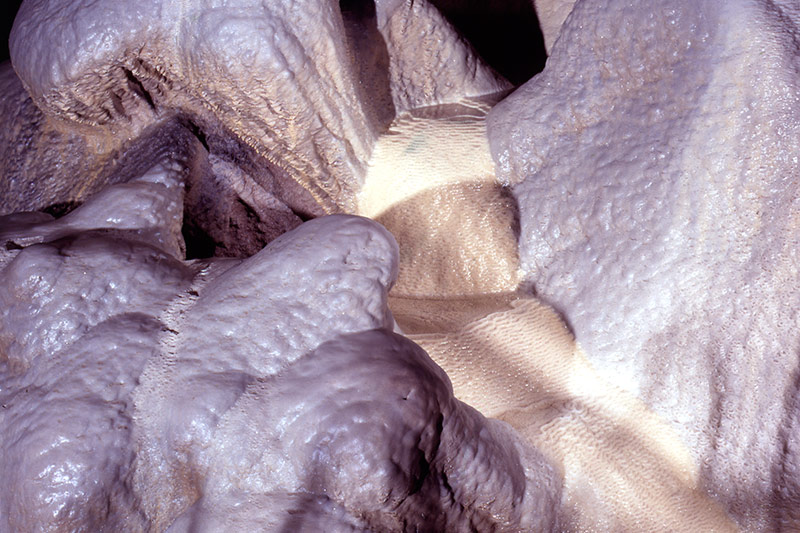
Spring water slowly flows through the cavern, eroding limestone / calcium deposits

Acidic rainwater reacting with limestone forms a solution of Calcium Bicarbonate (Ca (HCO3)2). This is commonly known as 'hard' water and forms the 'fur' seen on the elements of electric kettles.
As drips of rainwater form on the cave roof some Carbon Dioxide will discharge to the atmosphere causing some Calcium Bicarbonate to precipitate as Calcium Carbonate crystals, or Calcite. A slow drip of water will form a solid platelet crystal slowly forming a stalactite, whereas a comparatively fast drip will only deposit a hollow ring of Calcite creating a delicate hollow straw formation. Water splashing on the cave floor may leave Calcite to form stalagmites. The opposing formations sometimes grow together to make columns. Calcite also deposits as water runs down the cave rock faces creating flowstone and curtains. Gentler slopes may form into crystal pools with Calcite dams.
The variety of colours in Poole's Cavern are due to traces of minerals and impurities in the overlying rocks and soils. Red and orange hues may be due to traces of Iron compounds and organic material filtered through the rock pores. The blue/grey staining is thought to be caused by Manganese Dioxide from nearby shale deposits.
The most notable and unique formations in Poole's Cavern are the Poached Egg stalagmites, with their orange/red centre and white surround resembling the yolk of an egg. Recent studies have proved that these stalagmites and their associated stalactites grow at a considerably accelerated rate of up to 1cm per year, compared with the normal rate of several hundred years for the same amount of growth.
The industry of 18th Century Lime burning on Grinlow above the cavern created thousands of tons of fine dusty waste Lime powder, which was tipped above the Poached Egg chamber. This rich source of Calcite combined with rain water to form a concentrated Calcium Carbonate solution, causing stalactites and stalagmites to form in just a few decades. Proof of this can be easily seen where stalagmites are forming on the metal handrails.

These poached egg stalagmites grow at a considerably accelerated rate







